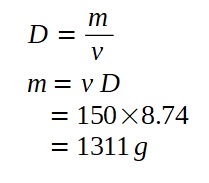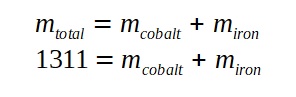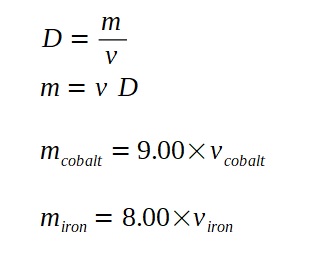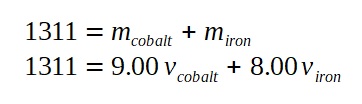Question
A molten alloy is made by mixing 450 g of molten cobalt of density 9.00 g/cm3 with 240 g of molten iron of density 8.00 g/cm3.
a) Calculate the density of the molten alloy in kg/m3. (An important assumption must be made to do this question.)
b) What is the assumption made in your calculation?
c)Suppose now a 150 cm3 molten alloy of cobalt and iron has a density of 8.74 g/cm3, calculate the volume of cobalt and iron in the alloy in cm3. (You may make the same assumption as in previous parts of the question.)
Answer
a) To find the density of the molten alloy, we need to find the total mass and total volume of the alloy.
We assume that the two metals do not react and that the total mass is the sum of their individual masses and the total volume is the sum of their individual volumes.
b) We assume that the two metals do not react and that the total mass is the sum of their individual masses and the total volume is the sum of their individual volumes.
c) To find the volumes of iron and cobalt, we first need to find the mass of the molten alloy.
We can then write down the relationships between the total mass and the mass of the cobalt and iron.
Similarly we can write down an expression for the volume of cobalt and iron.
Since we know the density of iron and cobalt, we can use the density to link the masses to their respective volumes.
Hence, the equation relating the total mass becomes:
We can now solve the following simultaneous equations: