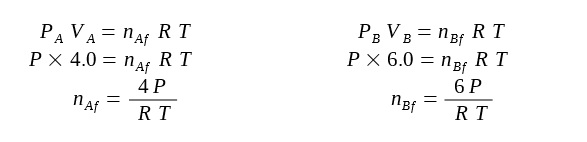Question
Two containers of volume 4.0m3 and 6.0m3 contain an ideal gas at pressures of 100Pa and 50Pa respectively. Their temperatures are equal. They are joined by a tube of negligible volume. The gas flows from one container to the other with no change in temperature. The final pressure will be
A) 70 Pa
B) 75 Pa
C) 80 Pa
D) 150 Pa
Answer
Before the valve is opened, we first find an expression for the initial number of moles of gas in each container:
Now, the total initial number of moles of gas can be found:
After the valve is opened, the pressure in each container is now the same and the temperature remains the same.
The new expression for the final number of moles in each container is found again:
Now, the total final number of moles of gas can be found:
Since the total number of moles remains the same,
Answer is A.